| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website http://www.wjnu.org |
Original Article
Volume 4, Number 2, June 2015, pages 207-212
Determination of Prooxidant and Antioxidant Balance, Clinical Parameters and Nutrient Intakes in Hemodialysis Patients
Farzaneh Montazerifara, Mansour Karajibanib, f, Mohammad Hashemic, Fatemeh Ardalid, Parisa Akramid, Ali-Reza Dashipoure
aPregnancy Health Research Center, Department of Nutrition, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran
bHealth Promotion Research Center, Department of Nutrition, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran
cDepartment of Clinical Biochemistry, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran
dStudent Scientific Research Center, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran
eDepartment of Nutrition, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran
fCorresponding Author: Mansour Karajibani, Department of Nutrition, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran
Manuscript accepted for publication February 16, 2015
Short title: Prooxidant and Antioxidant Balance
doi: http://dx.doi.org/10.14740/wjnu194w
| Abstract | ▴Top |
Background: There is evidence that hemodialysis (HD) leads to oxidative stress. However, reports about balance between oxidant and antioxidant are contradictory. Thus, the aim of this study was to evaluate the prooxidant-antioxidant balance (PAB) in HD patients and its correlation with clinical parameters and food intakes.
Methods: This case-control study was performed in 35 HD patients and 35 healthy controls. Food intake was assessed by a 24-hour dietary recall questionnaire for at least 2 days. Routine biochemical parameters and C-reactive protein (CRP) were measured using the standard techniques. PAB was simultaneously measured by a new modified PAB assay using 3,3ʹ,5,5ʹ-tetramethylbenzidine benzidine (TMB).
Results: PAB in HD patients was significantly higher compared with control group (88.2 ± 33 vs. 68.4 ± 23.3, P < 0.01). A significant positive correlation was established between PAB values and serum levels of creatinine (r = 0.38, P < 0.001), blood urea nitrogen (BUN) (r = 0.42, P < 0.0001) and CRP (r = 0.34, P < 0.001). An inverse correlation was found between PAB values and intake of vitamins A (r = -0.28, P < 0.05) and C (r = -0.31, P < 0.01) in HD patients.
Conclusion: Since little data exits on the PAB assay in the HD patients, the high values of PAB indicated in our study need to be confirmed by further studies.
Keywords: Prooxidant-antioxidant balance; Hemodialysis; Nutrient intakes
| Introduction | ▴Top |
The importance of oxidative stress in the pathogenesis of many diseases, including inflammatory, chronic and progressive diseases has been recently emphasized [1-4]. Oxidative stress can damage cellular components, such as lipids, proteins or DNA [2, 5]. There is evidence that suggests the increased state of oxidative stress in uremic patients on hemodialysis (HD) treatment may be related to disturbance in antioxidant mechanisms of free radicals detoxification [3, 6, 7]. The antioxidant defense system includes both endogenous and exogenous (food derived) antioxidants to protect cells against toxic effects [3, 8]. Dietary antioxidant micronutrients especially in fruits and vegetables may revive each other in an antioxidant system [3, 8]. Chronic stress in uremic patients on HD may be associated with disturbance in antioxidant defense mechanisms resulting from dietary restriction [3, 9, 10] or the direct effect of inflammation and macrophage activation caused by reactive oxygen groups during dialysis [11]. Some studies have indicated that increased oxidative stress in dialysis patients is linked to endothelial dysfunction, increased acute phase of inflammation, hyperlipidemia, and cardiovascular atherosclerotic diseases, which are the leading causes of morbidity and mortality in the HD patients [12-14]. A correct balance between oxidants and antioxidants may contribute to maintain the biological functions and reduce the risk of developing cardiovascular disease in patients with end-stage renal disease (ESRD) [3]. For the evaluation of the prooxidant-antioxidant balance (PAB), the measurement of both the oxidative damage markers and antioxidant levels is necessary. Despite the role of oxidative stress in patients on dialysis determination of PAB yet as a routine clinical laboratory methods are known. Many methods, separately measure the total antioxidant capacity and prooxidant, which is a complex and costly method [1, 2]. In this study, the oxidant and antioxidant capacity in an evaluation was measured. We also evaluated the relationship between PAB and lipid profile, C-reactive protein (CRP), and nutrient intakes in patients undergoing dialysis.
| Materials and Methods | ▴Top |
The study protocol was confirmed by the Ethics Committee of Zahedan University of Medical Sciences (2014-7-6862). Informed consent was obtained from all subjects participating in the study.
Subjects and study design
Thirty-five patients (mean age 43.1 ± 13.3 years, range 19 - 70 years) referring to Dialysis Section of Imam Ali Hospital in Zahedan who were dialyzed three times a week for at least 8 months with Kt/v ≥ 1.3, were enrolled in the study. The causes of ESRD included blood pressure (n = 16), kidney stone (n = 4), lupus erythematosus (n = 1), polycystic kidney (n = 4), glomerulonephritis (n = 4), and unknown (n = 6).
Thirty-five healthy volunteers (mean age 38.6 ± 12.5 years, range 19 - 69 years) free from any signs of kidney disease who were matched with the patients in terms of age and sex were selected as control group. Inclusion criteria for both two groups included age over 18 years, not using steroids and lipid lowering drugs, and lack of the active infection and inflammatory disease, liver disease, thyroid, cancer, as well as lack of hospitalization till 3 months before entering to study.
Anthropometric and dietary assessment
Dry weight after dialysis and height were measured. Body mass index (BMI) was evaluated based on the calculation of dry weight (kg)/height2 (m2) [15]. Food intake was assessed by a 24-h dietary recall questionnaire for at least 2 days. All of consumed food items were recorded on the two previous days. In order to avoid eventual differences between working and not-working days, the mean values of dietary intakes were measured on one weekday and one weekend. The questionnaire was filled in a personal interview that was conducted by trained investigators and a nutritionist. The nutrient values were calculated by CompEat nutritional analysis program [16].
Biochemical analysis
The blood samples were obtained from healthy subjects and before each infusion of heparin from each patient after a 12-h fast. Routine biochemical parameters including blood urea nitrogen (BUN), uric acid, creatinine, cholesterol, triglyceride, low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), serum albumin and CRP concentrations were measured using the standard techniques. The serum aliquots were immediately separated and frozen at -70 °C until analysis.
PAB assay
The PAB was measured by a modified method by Hamidi Alamdari et al [1, 17]. PAB values were assayed simultaneously, by photometric method, using 3,3ʹ,5,5ʹ-tetramethylbenzidine (TMB) in an ELISA reader at 450 nm (with a reference 570 nm or 620 nm wavelength). This method is based on two different reactions; in the enzymatic reaction the chromogen TMB is oxidized to a color cation by peroxides, and in the chemical reaction the TMB cation is reduced to a colorless compound by antioxidants. PAB is calibrated using a mixture of hydrogen peroxide and uric acid. The values are expressed in an arbitrary HK unit, which is as hydrogen peroxide percentage in the calibration mixture and demonstrates the oxidative stress index [1, 2, 17].
Statistical analysis
The results were expressed as mean ± SD and mean ± SEM as appropriate. Comparisons between two groups were analyzed by t-test or Mann-Whitney U-test. Pearson and Spearman correlation coefficients are used for assessment of parametric and non-parametric correlations. All statistical analysis was performed using SPSS software (version 18 for windows, Chicago, USA). A P value < 0.05 was considered significant.
| Results | ▴Top |
Demographic and clinical characteristics of participants are shown in Table 1. A significant difference was observed in BMI between HD patients and control subjects (P < 0.001).There was no significant difference in age and blood pressure levels between two groups.
 Click to view | Table 1. Demographic and Clinical Characteristics of Studied Groups |
Biochemical parameters are demonstrated in Table 2. The levels of BUN, serum creatinine (P < 0.0001), uric acid (P < 0.01) and CRP (P < 0.01) were markedly increased, and serum levels of albumin (P < 0.01) were significantly reduced in HD patients when compared to healthy controls. There was not any significant difference between two groups in other variables.
 Click to view | Table 2. Biochemical Parameters in the Studied Groups |
The PAB data analysis showed that PAB values in HD patients (88.2 ± 33) were significantly higher compared with control group (68.4 ± 23.3) (P < 0.01) (Fig. 1).
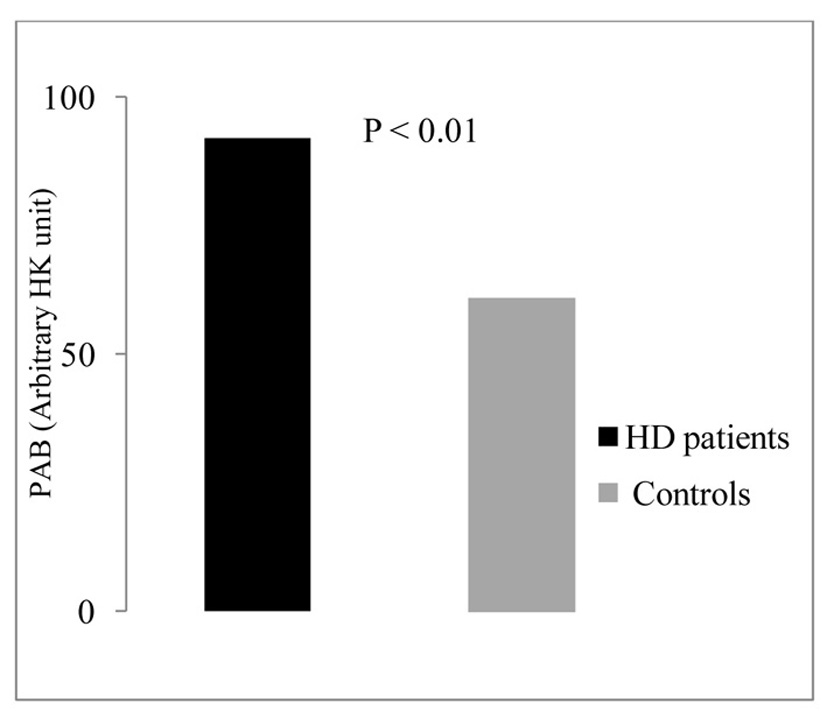 Click for large image | Figure 1. Serum levels of PAB in the studied groups. |
As shown in Table 3 [18, 19], intake of antioxidant vitamins including vitamins A, C and E (P < 0.01), and minerals including calcium (P < 0.01), phosphorous, potassium, zinc (P < 0.0001) and iron (P < 0.05) was significantly lower than control group.
 Click to view | Table 3. Nutrient Intakes in the Studied Groups [18, 19] |
A significant positive correlation was demonstrated between PAB value and serum levels of creatinine (r = 0.38, P < 0.001), BUN (r = 0.42, P < 0.0001) and CRP (r = 0.34, P < 0.001) in HD patients (Fig. 2). An inverse correlation between PAB value and intake of vitamins A (r = -0.28, P < 0.05) and C (r = -0.31, P < 0.01) was found (Fig. 3).
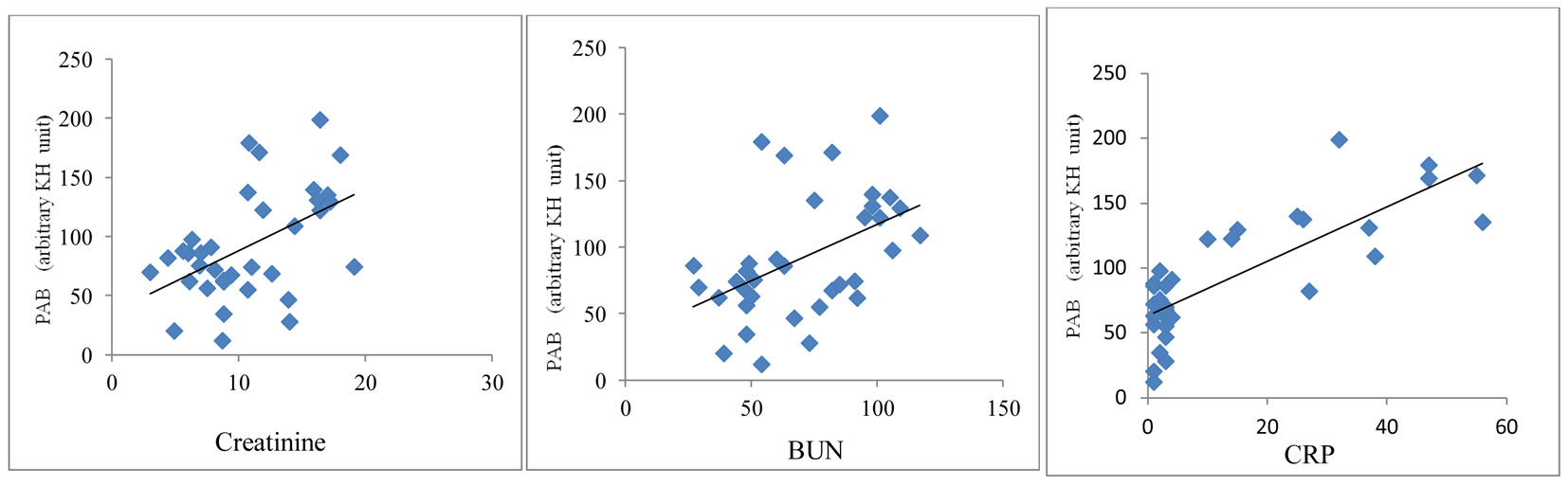 Click for large image | Figure 2. Correlation between PAB values and serum creatinine, BUN and CRP in HD patients. |
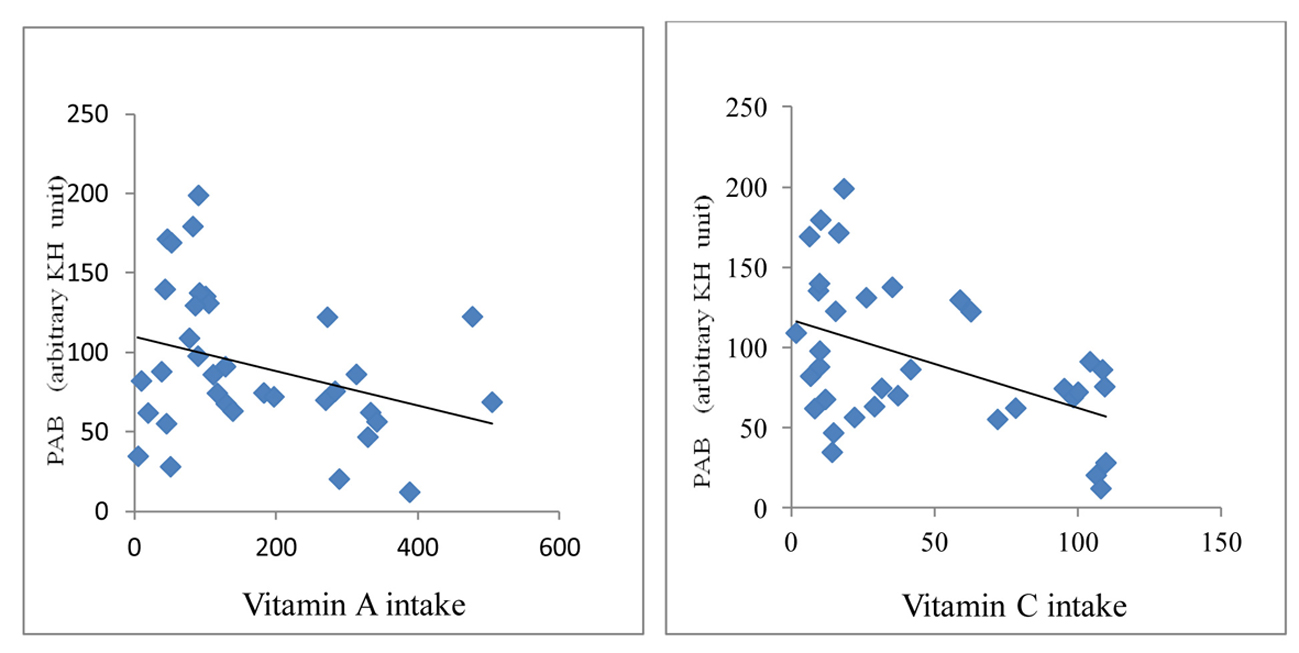 Click for large image | Figure 3. Correlation between PAB and vitamins A and C in HD patients. |
No statistically significant relationship was found between PAB value with dialysis duration, age, BMI, lipid profile and other micronutrients.
| Discussion | ▴Top |
To date, many methods have been performed to assay separately the prooxidant and antioxidant capacities, which are costly and complex. PAB assay using simple and rapid tests can help to identify patients at risk and prevent or reduce of complications resulting from oxidative stress. In our previous studies [6, 20], we indicated a defective antioxidant defense during the process of HD, due to the reduction of antioxidants and elevated lipid peroxidation in patients on HD in agreement with the other studies [7, 9, 12-14, 21]. However, a study has shown decrease in the serum levels of MDA compared to control group [22]. While in some studies, antioxidants activity [23] and lipid peroxidation have no change [24].
In the present study, we assayed simultaneously PAB assay values in HD patients. The findings demonstrated that PAB values were markedly higher in HD patients than healthy controls. There is evidence that both inflammation markers including CRP and tumor necrosis factor-α (TNF-α), and oxidative stress elevate the risk of chronic disease in uremic patients [9]. A significant positive correlation between PAB and serum levels of creatinine, BUN and CRP suggests that increasing the toxic nitrogen substances and inflammation in uremic patients undergoing HD can contribute to the oxidative stress, which plays an important role in the endothelial tissue injury and high risk of atherogenesis [3, 6, 12].
Moreover, the evidence suggests that dialysis therapy is also one of the causes of increased oxidative stress in uremic patients rather than the disease itself [25]. It could be attributed to the direct contact of blood with dialysis membrane [11], and/or the loss of the hydro-soluble vitamins through the dialysis membrane, which may contribute markedly to a reduced antioxidant power of plasma [3, 8, 26]. On the other hand, analysis of dietary intakes in our study showed that the dialysis patients consumed lower nutrients compared to controls, in particular intake of natural sources of antioxidant vitamins including A, E and C was significantly lower than controls as reported in our previous report [3]. A significant negative correlation between the PAB values and vitamins A and C intake was also found. It may be partly due to the inadequate intake of natural vitamins from fresh fruits and vegetables, nuts and seeds due to restriction of potassium and phosphate in these patients [3, 13]. Thus, efforts should be made to ensure that these patients receive optimum antioxidant vitamins. It seems that antioxidant therapy as a way to treat these patients should be considered.
Conclusion
The findings show that HD patients are exposed to oxidative stress. Since little information exits on the PAB assay in the HD patients, the high values of PAB demonstrated in our study need to be confirmed by further studies.
Acknowledgement
We are grateful to Mrs Shadi Amini, staff and nurses of Dialysis Section in Zahedan Imam Ali Hospital for their kind cooperation. We thank the patients and healthy subjects who willingly participated in the study.
Funding Source
This work was supported by the Research Deputy of Zahedan University of Medical Sciences, Zahedan, Iran, No. 6862.
Conflict of Interest
None.
| References | ▴Top |
- Alamdari DH, Paletas K, Pegiou T, Sarigianni M, Befani C, Koliakos G. A novel assay for the evaluation of the prooxidant-antioxidant balance, before and after antioxidant vitamin administration in type II diabetes patients. Clin Biochem. 2007;40(3-4):248-254.
doi pubmed - Boskabadi H, Moeini M, Tara F, Tavallaie SH, Saber H, Nejati R, Hosseini G, Mostafavi-Toroghi H, Ferns GAA, Ghayour-Mobarhan M. Determination of Prooxidant-Antioxidant Balance during uncomplicated pregnancy using a rapid assay. J Med Biochem. 2013;32(3):227-232.
doi - Montazerifar F, Hashemi M , Karajibani M, Dikshit M. Natural Antioxidants and Oxidative Stress Markers in Hemodialysis Patients. Hong Kong J Nephrol. 2010;12(2):57-61.
doi - Hekimi S, Lapointe J, Wen Y. Taking a "good" look at free radicals in the aging process. Trends Cell Biol. 2011;21(10):569-576.
doi pubmed - Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:5.
doi pubmed - Montazerifar F, Hashemi M, Karajibani M, Sanadgol H, Dikshit M. Evaluation of lipid peroxidation and erythrocyte glutathione peroxidase and superoxide dismutase in hemodialysis patients. Saudi J Kidney Dis Transpl. 2012;23(2):274-279.
pubmed - Galle J. Oxidative stress in chronic renal failure. Nephrol Dial Transplant. 2001;16(11):2135-2137.
doi pubmed - Prior RL, Cao G. In vivo total antioxidant capacity: comparison of different analytical methods. Free Radic Biol Med. 1999;27(11-12):1173-1181.
doi - Smith KS, Lee CL, Ridlington JW, Leonard SW, Devaraj S, Traber MG. Vitamin E supplementation increases circulating vitamin E metabolites tenfold in end-stage renal disease patients. Lipids. 2003;38(8):813-819.
doi pubmed - Galli F, Floridi AG, Floridi A, Buoncristiani U. Accumulation of vitamin E metabolites in the blood of renal failure patients. Clin Nutr. 2004;23(2):205-212.
doi - Handelman GJ. Evaluation of oxidant stress in dialysis patients. Blood Purif. 2000;18(4):343-349.
doi pubmed - Aguilera A, Bajo MA, Del Peso G, Diez JJ, Codoceo R, Rebollo F, Mariano M, Selgas R. True deficiency of antioxidancy of antioxidant vitamin E and A in dialysis patients. Relationship with clinical patterns of atherosclerosis. Adv Perit Dial. 2002:18:206-211.
pubmed - Varan HI, Dursun B, Dursun E, Ozben T, Suleymanlar G. Acute effects of hemodialysis on oxidative stress parameters in chronic uremic patients: comparison of two dialysis membranes. Int J Nephrol Renovasc Dis. 2010;3:39-45.
pubmed - Seyyedreza zadeh E, Ardalan MR, Pour moghaddam M. Serum lipid peroxidation and total antioxidant status in hemodialysis and continous ambulatory peritoneal dialysis patients. J Med Counc IR Iran. 2008;25(4):497-504. [Persian].
- Mahan LK, Escott-Stump S, Raymond JL. Krause's Food & the Nutrition Care Process, (Krause's Food & Nutrition Therapy), 13th edition. Philadelphia, Pa, USA: WB Saunders, Elsevier. 2012.
- Comp Eat, Nutritional Analysis Software. In: Nutrition Systems, Banbury, Oxon, England. 2003.
- Hamidi Alamdari D, Ordoudi S.A, Nenadis N, Tsimidou MZ, Koliakos G, Parizadeh MR, Safarian M, Sabery Karimian M, Nobakht BF. Comparison of prooxidant-antioxidant balance method with crocin method for determination of total prooxidant-antioxidant capacity. Iran J Basic Med Sci. 2009;12(2):93-99.
- Ribaya-Mercado JD, Solon FS, Fermin LS, Perfecto CS, Solon JA, Dolnikowski GG, Russell RM. Dietary vitamin A intakes of Filipino elders with adequate or low liver vitamin A concentrations as assessed by the deuterated-retinol-dilution method: implications for dietary requirements. Am J Clin Nutr. 2004;79(4):633-641.
pubmed - Hathcock JN, Azzi A, Blumberg J, Bray T, Dickinson A, Frei B, Jialal I, et al. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr. 2005;81(4):736-745.
pubmed - Montazerifar F, Hashemi M, Karajibani M, Dikshit M. Hemodialysis alters lipid profiles, total antioxidant capacity, and vitamins A, E, and C concentrations in humans. J Med Food. 2010;13(6):1490-1493.
doi pubmed - Siems W, Quast S, Carluccio F, Wiswedel I, Hirsch D, Augustin W, Hample Hm, Riehle M, Sommerburge O. Oxidative stress in chronic renal failure as a cardiovascular risk factor. Clin Nephrol. 2002;58(suppl 1):12-19.
- Daschner M, Lenhartz H, Botticher D, Schaefer F, Wollschlager M, Mehls O, Leichsenring M. Influence of dialysis on plasma lipid peroxidation products and antioxidant levels. Kidney Int. 1996;50(4):1268-1272.
doi pubmed - Durak I, Akyol O, Basesme E, Canbolat O, Kavutcu M. Reduced erythrocyte defense mechanisms against free radical toxicity in patients with chronic renal failure. Nephron. 1994;66(1):76-80.
doi pubmed - Erdogan C, Unlucerci Y, Turkmen A, Kuru A, Cetin O, Bekpinar S. The evaluation of oxidative stress in patients with chronic renal failure. Clin Chim Acta. 2002;322(1-2):157-161.
doi - Mayer B, Zitta S, Greilberger J, Holzer H, Reibnegger G, Hermetter A, Oettl K. Effect of hemodialysis on the antioxidative properties of serum. Biochim Biophys Acta. 2003;1638(3):267-272.
doi - Morena M, Cristol JP, Bosc JY, Tetta C, Forret G, Leger CL, Delcourt C, et al. Convective and diffusive losses of vitamin C during haemodiafiltration session: a contributive factor to oxidative stress in haemodialysis patients. Nephrol Dial Transplant. 2002;17(3):422-427.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.